Latest: Covid-19 booster shot: what's in it and how soon will you need it?
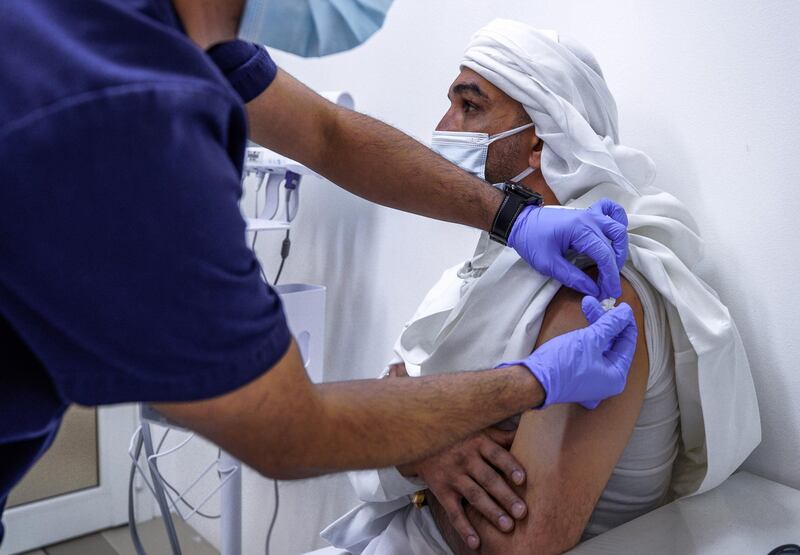
The Sinopharm vaccine is expected to offer people immunity against Covid-19 for four to six months, a top doctor involved in the UAE trial said.
Dr Nawal Al Kaabi, chairwoman of the National Covid-19 Clinical Management Committee, said Abu Dhabi's public health provider recorded a "significant decrease" in the rate of hospital admissions after the country began a mass vaccination campaign.
That was despite the second wave of the pandemic being worse than the first, she said.
"The number of hospitalisations was much less and there were no critical care admissions seen among the vaccinated, 14 days post second dose," Dr Al Kaabi said on Friday at an online public health conference about the Covid-19 response.
“There was no mortality among the vaccinated. Even for those who got the infection, usually it is mild or asymptomatic.
“We also noticed that the duration of positivity is very short among the vaccinated.”
The duration of immunity offered by two doses of the vaccine is likely to last six months or less, although she said further data was needed to determine the exact period.
“What is the best estimate for how long the immunity will last? Now, it is around four to six months, however, we don’t have the final data,” said Dr Al Kaabi, a paediatric infectious diseases consultant and chief medical officer at Sheikh Khalifa Medical City.

“There is a possibility that we will need a booster dose like any other inactivated vaccine.”
The conference also received an update about the Sputnik V vaccine trial in the UAE, which should begin a small Phase 2 study. It will test a combination of Sputnik V and AstraZeneca shots.
“The advantage here is at the time when we have a shortage of one or the other vaccine, this provides a new way to use combinations of different vaccines,” said Dr Ahmed Al Hammadi, a consultant in infectious diseases at Tawam Hospital in Al Ain.
Sputnik V was approved for emergency use in the Emirates on January 21 after the country hosted a small-scale Phase 3 trial of the vaccine involving about 1,000 volunteers. The shot was produced by Gamaleya National Centre of Epidemiology and Microbiology.
Clinical trial data shows a 91.4 per cent efficacy for the Sputnik V vaccine 28 days after the first dose is administered and more than 95 per cent after 42 days.
Trials placed Sinopharm's efficacy at 79.34 per cent, but some experts said it could hold up better against variants than other vaccine types.
That is because it is derived from the whole virus and does not only target the spike protein, where the most concerning mutations have been found in the variants.
Most of the other vaccines currently in use all target the spike protein.
But medics are still examining how effective vaccines are against new strains, including the Sinopharm shot.